Anzeige Regulatory consultation service for medical products registration industry policies. NMPA publishes guidelines for Device Master File DMF submissions.

36 of 2021 link in Chinese which outlines the requirements for the voluntary record filing of a Device Master File DMF for domestic Class III and imported Class II and III.

Master medical devices. The CBID medical device masters program familiarizes students with pre- and post-market requirements as well as device classifications import and export regulations and. Master of Science in Medical Device Engineering. Want to Master Medical Device Regulation learn how to put a product on the market in Europe.
Anzeige Regulatory consultation service for medical products registration industry policies. This programme focuses on core topics of Medical Device Design Manufacture Quality Systems Regulations Operational Excellence Emerging Trends Medical Measurement Strategic Thinking and Professional Skills. Courses range from taught MSc degrees to research oriented MRes and MPhil programmes.
A Master of Engineering Studies in Medical Devices and Technologies complements your background in engineering medicine or science with knowledge in medical devices technology and practice. This MS in Medical Device and Diagnostic Engineering program is designed to provide the knowledge and skills needed for the development of medical devices and diagnostic techniques including aspects of medical product regulation and of product development. Medical devices are products or equipment intended generally for a medical use and are regulated at Member State level.
The Medical Devices and the In-Vitro Diagnostic Devices Regulations have introduced new responsibilities for the European Medicines Agency EMA and national competent authorities in the assessment of certain categories of medical device. Technology development technology service technology transfer for medical products. Selling professional devices with added value services for our customer.
Learn how to choose your notified body or how the device regulation is different in other countries. Founded in 2011 Master Medical Equipment has always focused on one mission. Easy Medical Device is a platform for tools and resources for Regulatory Affairs Quality Management Regualtory Compliance.
Type of degree Master of Business Administration. Ihr technischer Dienstleister im Bereich der Medizintechnik. By scanning the globe to create lasting relationships with reputable manufacturers who in.
Technology development technology service technology transfer for medical products. The program prepares students for engineering roles in functional areas such as invention development and production of medical devices in specialties such as. Die Berufs- und Karrierechancen nach dem Abschluss sind exzellent.
Masters degrees in Medical Devices Instrumentation offer advanced training in the design and development of devices used to diagnose monitor treat and prevent diseases. This Master of Science in Medical Device Regulatory Affairs presents students with the opportunity to skills to employers and peers and to enhance your career in regulatory affairs by developing increasing levels of competence and professionalism. This industry-led Post-Graduate Diploma in Medical Device Technology Business is a unique blend of Scientific and Business Modules.
The Drug Master File DMF System allows the manufacturers of Active Pharmaceutical Ingredients APIs to submit the detailed information manufacturing methods data etc of APIs to the Review Authority PMDAThe registered information manufacturing methods data etc is quoted as the necessary information for an approval review of the pharmaceutical products in which APIs is used. Die DeviceMaster GmbH ist ein Zusammenschluss von erfahrenen klinischen regulatorischen und technischen Spezialisten die sich entschlossen haben ein junges und mehrsprachiges Projektteam aufzubauen um gemeinsam Dienstleistungen rund um die Zulassung von Medizinprodukten anzubieten. The course of study requires successful completion of 28 units of course work and has been.
The courses we offer are lecture-based delivered as modules. Imaging Medical Devices for Masters Students Imaging Medical Devices involves the measurement of spatial and temporal distributions and signals over scales ranging from molecules and cells to organs and whole populations. Der berufsbegleitende MBA-Studiengang Medical Devices Healthcare Management richtet sich an aufstiegsorientierte Mitarbeiter der Medizintechnik- und Biomedizintechnikbranche die ihre Kompetenzen im internationalen Management und Marketing ausbauen möchten.
Leadership staff at top medical device design companies such as Boston Scientific and Medtronic. Chinas National Medical Products Administration NMPA has published Announcement No. How can we do this.
The part-time Medical Devices Healthcare Management MBA programme is designed for career-oriented professionals in the fields of medical engineering and biomedical engineering seeking to improve their competencies in international management and marketing. Mit unserem konsekutiven Master-Studiengang Medical Devices - Research and Development MMD können Sie durch die Entwicklung und Verbesserung medizintechnischer Geräte einen Beitrag zum medizinischen Fortschritt leisten und so Verantwortung für die Zukunft und die Gesundheit des Menschen übernehmen. It is the gold standard in the field.
The MSMDE program at Keck Graduate Institute KGI in Claremont CA was created in response to the needs of the medical device industry.
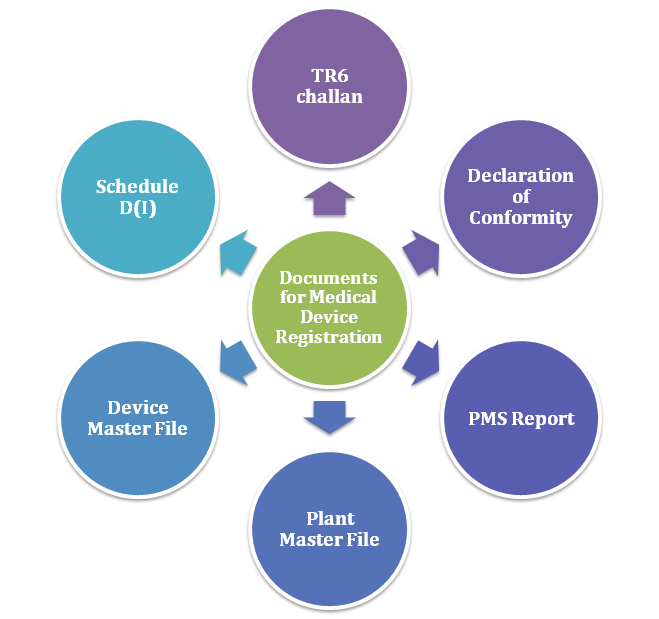 Know Checklist For Documents Required For Medical Device Registration
Know Checklist For Documents Required For Medical Device Registration
 Medical Devices Validation Manager European Compliance
Medical Devices Validation Manager European Compliance
 Master Program In Medical Devices Engineering In Costa Rica Journal Of Engineering In Medical Devices
Master Program In Medical Devices Engineering In Costa Rica Journal Of Engineering In Medical Devices
 Active And Non Active Medical Devices Cn Tuv Rheinland
Active And Non Active Medical Devices Cn Tuv Rheinland
 Master Data Management Platform For Medical Devices Manufacturers By Riversand Issuu
Master Data Management Platform For Medical Devices Manufacturers By Riversand Issuu
 Master Management Medical Devices Healthcare Management Mba
Master Management Medical Devices Healthcare Management Mba

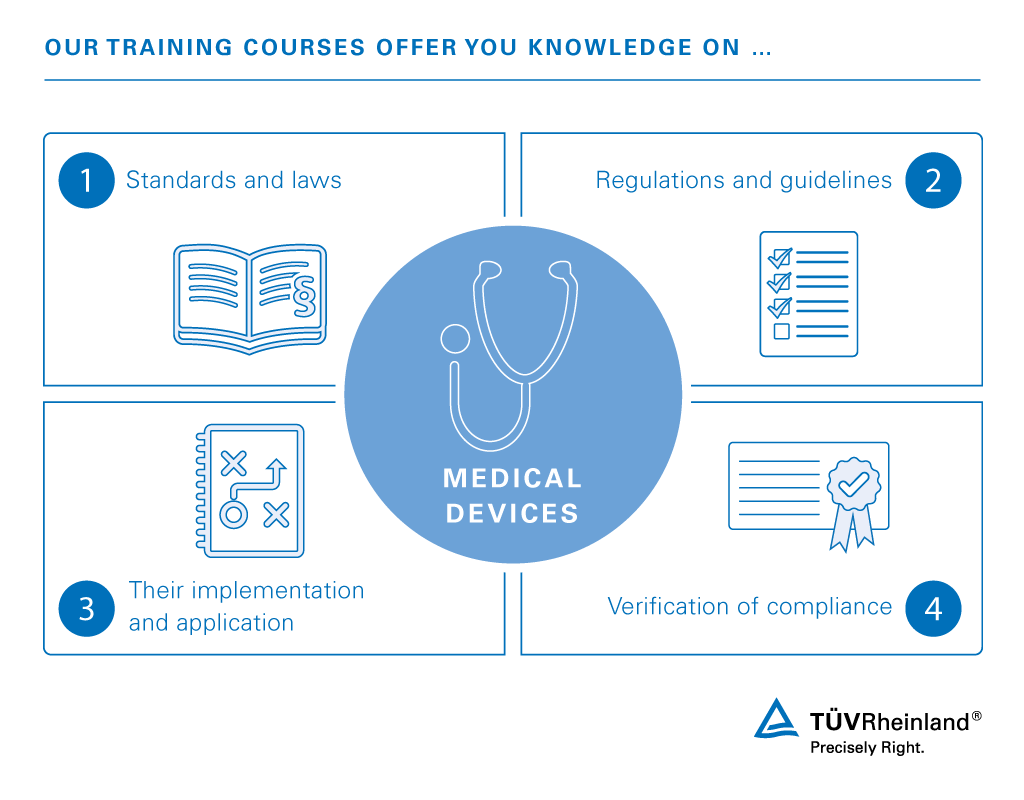 Medical Device Training Iq Tuv Rheinland
Medical Device Training Iq Tuv Rheinland
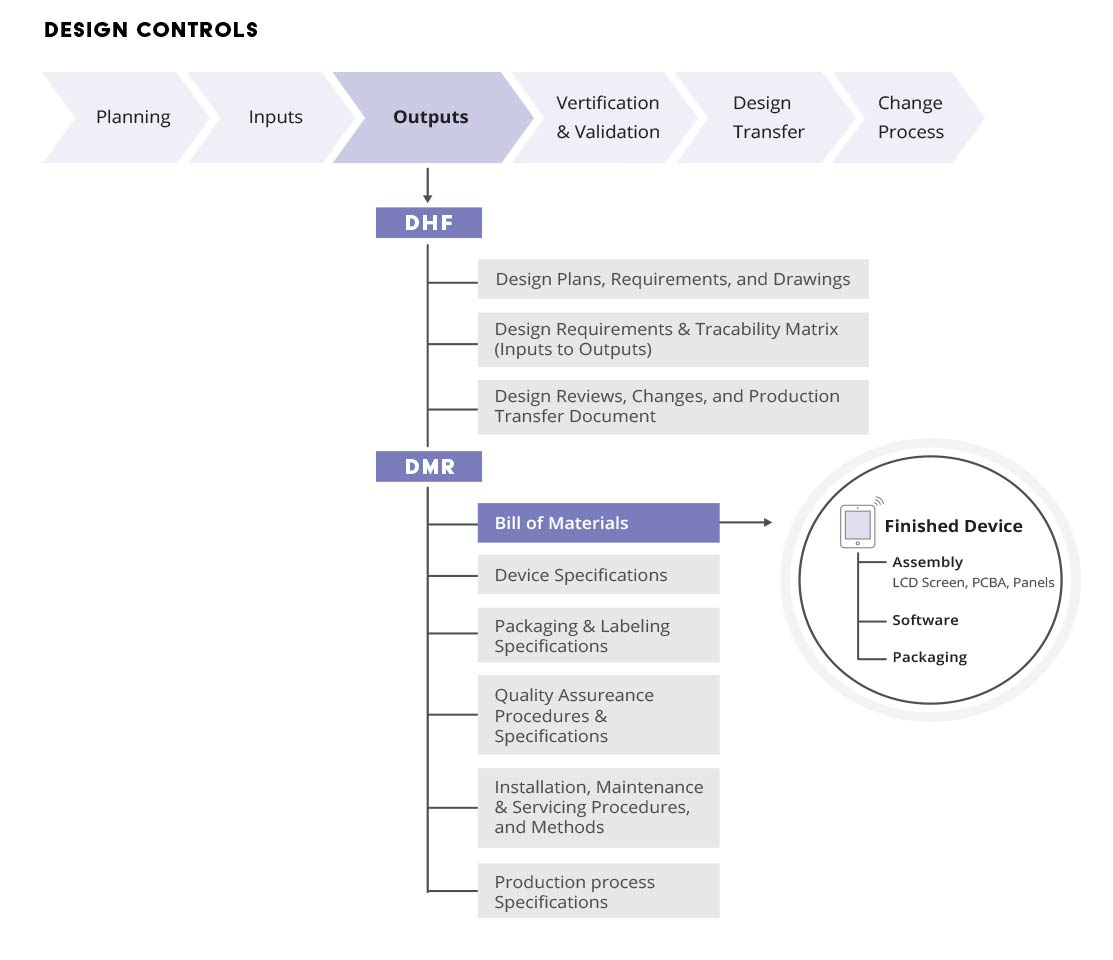 Managing The Device Master Record Dmr To Comply With 21 Cfr Part 11 And Part 820 Arena
Managing The Device Master Record Dmr To Comply With 21 Cfr Part 11 And Part 820 Arena
 New Refurbished Used Medical Equipment Mme
New Refurbished Used Medical Equipment Mme
 Non Active Medical Device Testing Tr Tuv Rheinland
Non Active Medical Device Testing Tr Tuv Rheinland
 Latest Innovative Report On Medical Device Process Validation Services Market By 2026 With Top Key Players Like Emergo Master Control Operon Strategist Bioteknica Bmp Medical Ksu The Sentinel Newspaper
Latest Innovative Report On Medical Device Process Validation Services Market By 2026 With Top Key Players Like Emergo Master Control Operon Strategist Bioteknica Bmp Medical Ksu The Sentinel Newspaper
 Medical Devices For The Eu 070910
Medical Devices For The Eu 070910
 Medical Products Mdd Wo Tuv Rheinland
Medical Products Mdd Wo Tuv Rheinland

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.