Tecfidera dimethyl fumarate is a prescription medicine used to treat relapsing forms of multiple sclerosis in adults including clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease. Dimethyl fumarate is sometime abbreviated as DMF and is sold under the brand name Tecfidera.
Click here to read the Tecfidera Prescribing Information for healthcare professionals.
Tecfidera prescribing information. 2 DOSAGE AND ADMINISTRATION. FULL PRESCRIBING INFORMATION 1 INDICATIONS AND USAGE. TECFIDERA DIMETHYL FUMARATE 7.
Dimethyl fumarate is a prescription medication used to treat a type of multiple sclerosis with symptoms that flare up from time to time known as relapsing-remitting multiple sclerosis. Click here to read the Tecfidera Patient Information. Tecfidera is approved by the FDA for the treatment of relapsing forms of multiple sclerosis to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in adults.
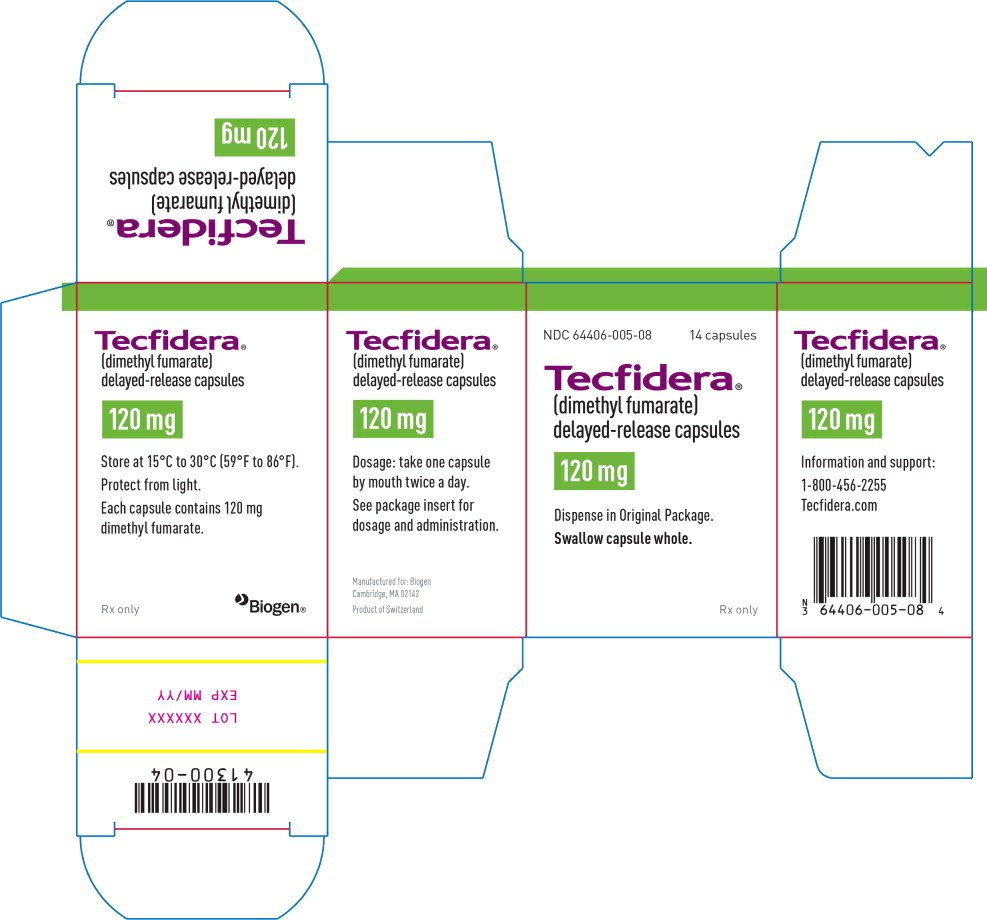
The prescribing information for the oral multiple sclerosis MS medication dimethyl fumarate Tecfidera Biogen has been updated to include. 42 Posology and method of administration Treatment should be initiated under supervision of a physician experienced in the treatment of. Patient Information TECFIDERA tek fi de rah dimethyl fumarate delayed-release capsules.
For full Prescribing Information including Patient Information click here. What is TECFIDERA dimethyl fumarate. TecTrack is an app brought to you by Biogen to help you keep track of your Tecfidera dimethyl fumarate treatment.
TECFIDERA is indicated for the treatment of patients with relapsing forms of multiple sclerosis. In 2017 the prescribing information for Tecfidera was amended to include direction to obtain a complete blood cell count and to measure liver enzymes and other values before initiating the medication. Download the application to.
Tecfidera dimethyl fumarate was approved by the FDA on March 27 2013 to treat adults with relapsing forms of MS. TECFIDERA is a prescription medicine used to treat relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in adults. Tecfidera dimethyl fumarate is a brand-name prescription drug thats used to treat relapsing forms of multiple sclerosis MS.
Warnings and other important changes have been added to the prescribing information for Tecfidera dimethyl fumarate Biogen Idec. Tecfidera is a prescription medicine used to treat relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in adults. Get reminders to take your TECFIDERA and get your bloodwork done Log each TECFIDERA dose you take Customize your alarms Track common side.
While its important to work with a healthcare provider to figure out what works best for you weve shared some tips that may help manage common side effects of TECFIDERA. TECFIDERA is indicated for the treatment of relapsing forms of multiple sclerosis MS. 1 INDICATIONS AND USAGE.
Tecfidera is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis see section 51 for important information on the populations for which efficacy has been established. After 7 days a maintenance dose of 240 mg twice a day is recommended. About TECFIDERA dimethyl fumarate TECFIDERA a treatment for relapsing forms of multiple sclerosis MS in adults is the most prescribed oral medication for relapsing MS in the world and has.
We know that dealing with side effects can be challenging. Patient Information Tecfidera tek fi de rah dimethyl fumarate delayed-release capsules. About TECFIDERA dimethyl fumarate TECFIDERA a treatment for relapsing forms of multiple sclerosis MS in adults is the most prescribed oral medication for relapsing MS in the world and has.
Its classified as a disease-modifying therapy. The recommended starting dose of dimethyl fumarate is 120 mg orally twice a day for 7 days. FULL PRESCRIBING INFORMATION.
Tecfidera is an oral disease-modifying therapy for adults with relapsing forms of multiple sclerosis. - TECFIDERA is a prescription medicine used to treat relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in adults - It is not known if TECFIDERA is safe and effective in children under 18 years of age.
 3rd Oral Drug To Treat Ms Is Approved By The F D A The New York Times
3rd Oral Drug To Treat Ms Is Approved By The F D A The New York Times
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2021 204063s026lbl Pdf
 Tecfidera Fda Prescribing Information Ingredients Mechanism Of Action
Tecfidera Fda Prescribing Information Ingredients Mechanism Of Action
 These Highlights Do Not Include All The Information Needed To Use Tecfidera Safely And Effectively See Full Prescribing Information For Tecfidera Tecfidera Dimethyl Fumarate Delayed Release Capsules For Oral Useinitial U S Approval 2013
These Highlights Do Not Include All The Information Needed To Use Tecfidera Safely And Effectively See Full Prescribing Information For Tecfidera Tecfidera Dimethyl Fumarate Delayed Release Capsules For Oral Useinitial U S Approval 2013
Https Healthy Kaiserpermanente Org Content Dam Kporg Final Documents Formularies Nw Tecfidera Nw En Pdf
 Buy Tecfidera Dimethyl Fumarate Price Costs Thesocialmedwork
Buy Tecfidera Dimethyl Fumarate Price Costs Thesocialmedwork
 Tecfidera Fda Prescribing Information Ingredients Mechanism Of Action
Tecfidera Fda Prescribing Information Ingredients Mechanism Of Action
Fda Adds Rare Brain Infection Link To Tecfidera Labelling Pmlive
 European Neurologists Expect Biogen Idec S Tecfidera To Be Preferred Ms Drug
European Neurologists Expect Biogen Idec S Tecfidera To Be Preferred Ms Drug

Https Www Tecfidera Com Content Dam Commercial Tecfidera Pat En Us Pdf Questions When Considering Treatment Pdf
 Tecfidera Fda Prescribing Information Ingredients Mechanism Of Action
Tecfidera Fda Prescribing Information Ingredients Mechanism Of Action
 Rx Item Tecfidera 240mg Cap 60 By Biogen
Rx Item Tecfidera 240mg Cap 60 By Biogen

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.